Abstract
Background: Monoclonal gammopathy of undetermined significance (MGUS) is a precursor of multiple myeloma and related conditions. Previous registry-based studies and retrospective clinical case studies have proposed an increased risk of MGUS among patients with autoimmune diseases. However, as the clinical diagnosis of MGUS is, by definition, made when investigating the cause of unrelated symptoms, prior studies have been unable to control for selection bias. Therefore, the true relationship between MGUS and autoimmune diseases remains unknown and can only be evaluated in a screening study.
Aims: To examine whether autoimmune diseases are associated with MGUS in a screened population.
Methods: This study used data from Iceland Screens, Treats, or Prevents Multiple Myeloma (iStopMM), a population-based screening study of MGUS. Briefly, 93% of 80,759 consented participants, representing 54% of the Icelandic population aged 40 years or older, have been screened for MGUS using serum protein electrophoresis (SPEP) and a free light chain (FLC) assay. To identify individuals in the study with a previous clinical diagnosis of MGUS, records from the two laboratories performing SPEP in Iceland were used and cross referenced with the Icelandic Cancer Registry. Diagnoses of autoimmune diseases were extracted from the Icelandic Hospital Discharge Register and the Icelandic Register of Primary Health Care Contacts, both of which have >95% completeness and accuracy. All diagnoses from the 1st of January 1999 to 31st of December 2020 were included. T-test and chi-squared test were used for univariable comparison of the prevalence of autoimmune disease among those without any MGUS (whether clinical or screened, the control group), compared with different groups: individuals with a positive screening test for MGUS and those with a previous clinical diagnosis of MGUS, as well as both groups combined. Logistic regression was used to calculate odds ratios (OR) with 95% confidence intervals (CI), adjusting for age and sex, comparing the same two groups to the control group as in the univariable analysis.
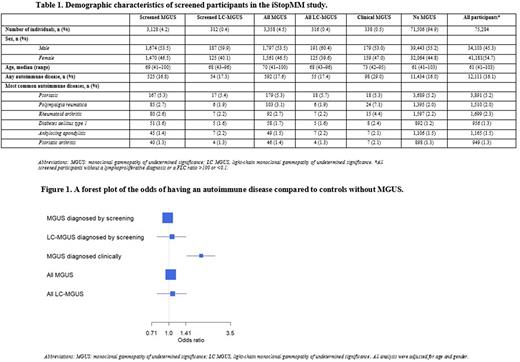
Results: Among 75,422 screened samples 3,358 had MGUS and 316 light chain MGUS (LC-MGUS). Of all screened individuals, 338 had a prior clinical diagnosis of MGUS or LC-MGUS and 234 had both clinical and screened MGUS, 104 were negative at screening. Individuals with MGUS were older (median age 70 vs 61, p<0.001) than those without MGUS and more frequently male (53.5% vs 45.3%, p<0.0001). Of all participants, 12,111 (16.1%) had a diagnosis of an autoimmune disease, whereof psoriasis, rheumatoid arthritis, polymyalgia rheumatica, ankylosing spondylitis, and diabetes mellitus type 1 were most common (Table 1). In the screened population, neither MGUS nor LC-MGUS were associated with autoimmune disease compared with individuals without MGUS or LC-MGUS (controls), OR 0.98 (95% CI 0.89-1.08) and 1.07 (95% CI 0.79-1.42), respectively. However, among individuals with a prior clinical diagnosis of MGUS, we found an association with autoimmune disease, OR 1.93 (95% CI 1.44- 2.56) (Figure 1). When the clinical and screened MGUS groups were combined the results were similar to the screened group alone (Figure 1).
Conclusion: In this large, prospective, population-based screening of over 75,000 individuals, we did not find an association between MGUS or LC-MGUS and autoimmune disease. However, in sharp contrast, we did find a correlation with autoimmune disease among individuals diagnosed with MGUS in a clinical setting. Our results support the hypothesis that clinical case series and prior registry-based studies showing an increased risk of MGUS among patients with autoimmune diseases reflect a detection bias. This large, prospective screening study negates the previously reported biological correlation between autoimmune disease, MGUS and LC-MGUS and shows that autoimmune diseases are not associated with MGUS.
Disclosures
Hultcrantz:Intellisphere LLC: Consultancy; Curio Science LLC: Consultancy; Amgen, Daichii Sankyo, Cosette, GSK: Research Funding; Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees; GSK: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Durie:Amgen: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Celgene/BMS: Consultancy, Honoraria; Janssen: Consultancy, Honoraria. Harding:The Binding Site: Current Employment, Membership on an entity's Board of Directors or advisory committees. Landgren:Merck & Co., Inc.: Other: Independent Data Monitoring Committee (IDMC) member for clinical trials; Janssen: Honoraria, Other: Independent Data Monitoring Committee (IDMC) member for clinical trials, Research Funding; Riney Foundation: Research Funding; NCI/NIH: Research Funding; Theradex: Other: Independent Data Monitoring Committee (IDMC) member for clinical trials; Pfizer Inc: Consultancy; Aptitude Health: Honoraria; Tow Foundation: Research Funding; Rising Tide Foundation: Research Funding; Leukemia & Lymphoma Society: Research Funding; MMRF: Honoraria; Amgen: Honoraria, Research Funding. Kristinsson:Amgen: Research Funding; Celgene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.